Chlorination
In this article we discuss about Chlorination
After reading you will be able to answer
1) what is Chlorination
2) why Chlorination
3) forms of chlorine address
4) favourable conditions for chlorination,etc
Like share and follow
Let's start 👇
 |
| Chlorination |
(HOCl OCl andCl ) are combined
called freely available chlorine. Out of
these forms of freely available chlorine,
HOCl is most destructive. It is 80%
more effective than OCl- ion Hence pH
of water should be maintained slightly
below 7.
Moreover chlorine will immediately
react with ammonia present in water to
form chloramines.
 |
| Chloramines |
Chloramines are combined form of
chlorine. It is less effective than free
chlorine (25 times lesser). But they are
stable and remain in water for greater
duration.
In the usual chlorine treatment, in
which PH is kept slightly less than 7,
dichloramine is most predominate.
These disinfectant kill those enzymes
which are essential for the metabolic
process of living organism.
Doses of chlorine should be sufficient so
as to leave a residue of 0.2 mg per litre
after 10 minutes of contact period. This
does is called chlorine demand of water.
The residual chlorine is tested by DPD
(Diethyl-Paraphenylene diamine) test
FORMS IN WHICH CHLORINE IS ADDED
a) free chlorine (liquid or gaseous form)
b) Hypochlorite’s (Bleaching powder)
c) Chloramines (ammonia + chlorine)
d) Chlorine dioxide (ClO2)
FREE CHLORINE
Liquid form is mostly used.
If temperature is below 10°C, liquid
chlorine will get frozen into ice crystals
which will stick and choke the lines of
feeding. Hence liquid cylinder is kept
at 32 - 48°C.
Steel cylinder burns in dry chlorine at
temperature greater than 92°C. Hence
high temperature is avoided.
Chlorine forms explosive mixture with
carbon monoxide.
Chlorine is applied through an
equipment called chlorinator.
Free Chlorine can be stored for long
time without being deteriorated.
Chlorine dose can be easily measured in
liquid forms. Hence under loading and
overloading is less frequent. Chlorine is
a powerful disinfectant and remains in
water for a long time when ammonia is
present.
No Sludge is formed in its application as
may be produced in Hypochlorites and
chloramines.
HYPOCHLORITE OR BLEACHING
OCl-and -HOCl are the disinfectant in
this case. This process is called hypochlorination.
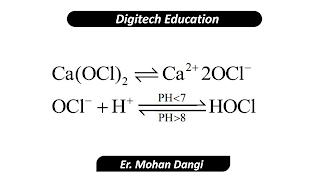 |
| Bleaching powder |
100% pure hypochlorite should contain
free available chlorine equal to OCl
value of the compound.
E.g. 142 gm of Ca(Clo)2 will contain 102 gm of OCl
i.e.
free available chlorine in 100% pure
calcium hypochlorite is 70%. But the
actual chlorinating ability is lesser
because bleaching powder is unstable
and goes on losing its chlorine content
when exposed to atmosphere.
Hypochlorites are generally not used in
modern days because they increase pH
because to they are having lime content.
Hypochlorite contains very low amount
of chlorine.
It is used for swimming pools only.
USE OF CHLORAMINES
Chloromines are weaker disinfectants
(25 times lesser than chlorine). Hence
either higher dose or longer contact
period is used.
Chloramines are stable and can remain
in water for a long time contrary to
unstable chlorine which evaporates
after some time. Hence they provide
greater safeguard against future
pollution.
They are weaker as compared to free
chlorine but do not cause bad taste
when left as residue.
When phenol is present in water
chloramines are mostly used because
chlorine with phenols gives bad taste.
But chloramines with phenol does not
give any taste.
For producing chloramines ammonia is
added to filtered water before adding
chlorine.
Amount of ammonia should be
1/3 to 1/4 of the amount of chlorine. They are added in water and mixed for 20
minutes to 2 hours before adding
chlorine. This contact period of
ammonia should be higher when phenol
is present.
Ammonia adding instrument is called
ammoniator.
CHLORINE DIOXIDE (ClO2)
It is highly effective (2.5 times stronger than free chlorine).
ClO2 is highly unstable hence should
be used immediately after production
It may also be used when phenol is
present and can also remove organic
impurities.
pH range is 8 – 10.
Normal dose is 0.5 – 1.5 mg/litre










0 Comments